# Essential Clinical Project Manager Templates You Need (2026 Edition)
When you’re knee-deep in managing a clinical trial in 2026, having the right templates can make all the difference. They’re like your trusty roadmap, guiding you through the twists and turns of project management. From planning and tracking to communicating and assessing risks, these templates help keep everything in check. Without them, you’d probably feel like you’re trying to build a house without a blueprint—messy and chaotic.
So, let’s dive into the world of clinical project manager templates and see how they—and platforms like Gridfox—can make your life a whole lot easier.
Hey, ever wondered why templates are such a big deal in clinical trials? Well, they’re basically your best mate when it comes to keeping everything on track. Imagine trying to juggle a million things without a plan. Templates bring structure to the chaos, making sure you don’t miss a step. They help you outline the scope, set goals, and keep everyone on the same page.
In clinical trials, a well-structured template—especially when digitised in a tool like Gridfox—can be the difference between smooth sailing and complete mayhem.
Now, let’s chat about how templates make project management a breeze. They’re like a roadmap for your trial, showing you where to go next. With templates, you can plan your tasks, manage resources, and track progress without breaking a sweat. Plus, they let you focus on what really matters—keeping the trial running smoothly and hitting those milestones.
Here’s how they help:
Consistency:
Ensures every trial follows the same process, reducing errors.
Efficiency:
Saves time by using pre-defined formats and structures.
Clarity:
Makes it easier for everyone to understand their roles and responsibilities.
So, what makes a template effective? Well, it should be user-friendly and adaptable to different trials. You want something that’s easy to tweak as your project evolves. Flexibility is key here. Effective templates also include clear instructions and sections for every aspect of your trial—from risk assessment to stakeholder communication.
Here’s a quick checklist:
Using the right templates can turn a challenging clinical trial into a well-oiled machine. And let’s face it, anything that makes the process easier is a win in our book!
Creating a solid Project Management Plan (PMP) for clinical trials is like setting up a roadmap for your research journey. It outlines everything from your objectives to how you’ll handle hiccups along the way. Here’s what you should include:
Project Objectives:
Clearly state what you aim to achieve. This isn’t just about the end goal but also the standards you’ll use to measure success.
Background and Strategic Context:
Lay down the groundwork by explaining the context of your study. This helps in making informed decisions as the project progresses.
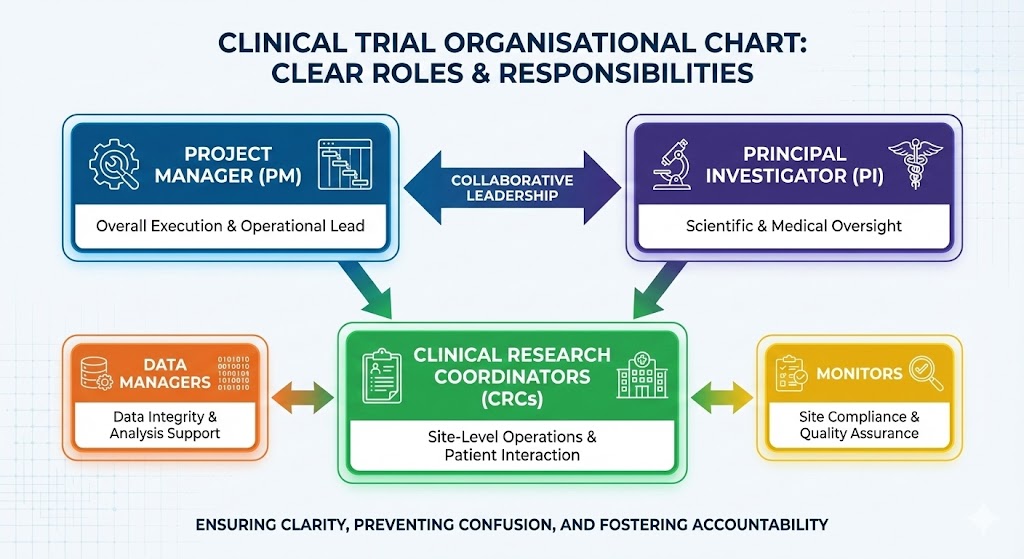
Study Governance:
Define roles and responsibilities to keep communication open and ensure accountability.
Stakeholder Management Plan:
Detail how you’ll keep everyone in the loop—whether through emails, newsletters, or meetings.
Scope:
Identify what’s included in your project, any assumptions, constraints, and deliverables.
Project Risk Assessment:
Prepare for potential risks and decide on your risk tolerance.
Using a PMP template can save you a tonne of time and effort. Here’s why:
Consistency:
Templates ensure that all your projects follow the same format, making it easier to track progress and compare different studies.
Efficiency:
With a template, you don’t start from scratch each time. Just fill in the specifics for each trial.
Clarity:
A well-structured template helps in clearly communicating the plan to all stakeholders.
Not all trials are the same, so your PMP shouldn’t be either. Customising allows you to:
Customising your PMP is like tailoring a suit; it must fit the project perfectly to be effective. Gridfox allows you to build these custom fields directly into your project management boards, meaning you don’t have to shy away from tweaking your plan to meet the unique demands of your clinical trial.
Tracking templates are like the backbone of any clinical research project. They help you keep tabs on every moving part, from patient enrolment to data collection. Let’s break down the types and how to use them effectively.
Case Report Forms (CRFs):
These are crucial for gathering data on each participant. You can use them in both paper and electronic formats, making data collection flexible.
Regulatory Binders:
These keep all essential documents organised and easily accessible. Think of them as your project’s filing cabinet.
Document Tracking Logs:
These logs help you track the submission and approval of critical trial documents, ensuring nothing falls through the cracks.
Using tracking templates effectively can be a game-changer. Here’s how you can make the most of them:
Consistency is Key:
Always update your templates regularly. This keeps your data accurate and prevents any last-minute scrambles.
Train Your Team:
Make sure everyone knows how to use these templates. A little training goes a long way in avoiding errors.
Customise for Your Needs:
Tailor your templates to fit the specific requirements of your trial. This makes them more relevant and useful.
Digital tracking tools offer a modern twist to traditional templates. They bring several advantages to the table:
Real-Time Updates:
With digital tools like Gridfox, data can be updated in real-time, reducing the risk of outdated information.
Enhanced Collaboration:
These tools often come with features that allow multiple team members to access and update information simultaneously.
Improved Data Security:
Digital platforms often include security features that protect sensitive data from unauthorised access.
Embracing digital tracking in clinical research not only streamlines processes but also enhances accuracy and compliance. It’s about making your life easier while ensuring the integrity of your data. For those looking to streamline their project management further in 2026, consider exploring Gridfox’s Project Manager Template, which offers a variety of tools tailored to enhance productivity and organisation.
Keeping everyone in the loop is key, right? That’s where templates for stakeholder communication come in. They help you organise and streamline all those emails, updates, and reports. With a good template, you can ensure that everyone gets the right info at the right time. It’s like having a personal assistant that never sleeps!
Ever had a stakeholder who expected the moon? Managing expectations can be tricky, but templates can help set the right tone from the start. They provide a structured way to outline what’s possible and what’s not. Use them to lay down the facts, timelines, and deliverables, so there’s no room for misunderstandings.
In the world of clinical trials, communication tools are your best mates. They not only keep the team connected but also ensure that stakeholders are always informed. Here’s a quick list of tools that can make your life easier:
Email Templates:
For regular updates and announcements.
Project Management Software:
To track progress and share updates automatically.
Instant Messaging Apps:
For quick, informal chats.
Using the right tools and templates can transform how you communicate. Gridfox, for instance, allows you to centralise these communications alongside your project data, making sure everyone knows what’s happening and when.
When you’re knee-deep in a clinical trial, it’s crucial to spot potential risks early on. A clear and detailed risk management plan is essential for assessing the impact of project risks and understanding the potential outcomes of decisions. Think about anything that might derail your project, like unexpected side effects or recruitment issues. Listing these risks upfront can save a lot of headaches down the line.
Risk assessment templates are your best mates in this process. They help you organise risks into categories, like operational or financial, so you can tackle them systematically. With a risk register template—easily built within Gridfox—you can track each risk’s likelihood and impact, ensuring nothing slips through the cracks.
Once you’ve identified the risks, it’s time to figure out how to handle them. Here are a few strategies:
Avoidance:
Change your plan to sidestep the risk.
Mitigation:
Reduce the impact or likelihood of the risk.
Transfer:
Shift the risk to a third party, like through insurance.
Acceptance:
Sometimes, you just have to accept the risk and plan for its potential impact.
Taking a proactive approach to risk management can make all the difference in the success of a clinical trial. By using templates, you’re not just preparing for the worst; you’re setting your project up for success.
Alright, so you’re diving into the world of budgeting for clinical trials. It’s not just about crunching numbers; it’s about making sure every penny is well spent. Using templates can be a lifesaver. They help you organise costs, forecast expenses, and keep everything on track. You can find templates that let you:
Imagine having a digital table where you can plug in numbers and see where you’re overspending or where you might have a bit of wiggle room. That’s the power of a good budget template.
Managing resources in clinical trials involves more than just money. You’ve got people, equipment, and time on your hands. A solid resource plan template can help you:
Think of it as a roadmap that guides you through the trial process, ensuring everything and everyone is where they need to be.
Now, let’s talk about getting the most out of your resources. With template tools, you can optimise everything from staff schedules to equipment usage. Here’s how:
Use
Gantt charts
to visualise project timelines and resource allocation.
Implement
Kanban boards
to track task progress and resource availability.
Develop
contingency plans
for unexpected resource shortages.
Gridfox offers built-in Gantt and Kanban views that make this process seamless. Remember, the goal is to maximise efficiency without burning out your team or blowing the budget. Templates make it easier to plan, adjust, and succeed in your clinical projects.
Wrapping up a clinical trial isn’t just about collecting data and calling it a day. It’s about ensuring every piece of information is captured, documented, and stored correctly. This is where post-study documentation comes in. It’s your responsibility to make sure everything is in order, from investigational product logs to close-out reports. This documentation is essential for compliance and future reference. Without it, you might find yourself in a tricky spot if regulators come knocking.
Templates can make your life a whole lot easier when summarising clinical studies. They provide a structure that ensures you don’t miss out on any critical details. A typical summary report might be a couple of pages long, highlighting the key findings and outcomes of your trial. Think of it as a snapshot that gives a quick overview without diving into the nitty-gritty. It’s a handy tool for communicating results to stakeholders, whether they’re sponsors or the public.
Compliance isn’t just a buzzword—it’s a necessity in clinical research. Using the right templates can help you stay on top of regulatory requirements. They guide you through the process, making sure you tick all the boxes and follow the necessary steps. Templates for accountability logs, destruction forms, and close-out checklists are just a few examples. They help you keep track of everything, from drug stock to final reports, ensuring nothing slips through the cracks.
So there you have it, a bunch of templates that can really make a difference in managing clinical projects in 2026. Whether you’re just starting out or have been in the game for a while, having the right tools can save you a lot of headaches. It’s like having a map when you’re lost in a new city. You might still take a wrong turn here and there, but at least you have something to guide you back on track.
Remember, every project is unique, but the basics of good management don’t change. Use these templates as a foundation—and consider digitising them with Gridfox to fit your specific needs—and you’ll be well on your way to running smoother, more efficient trials. And who knows, maybe you’ll even enjoy the process a bit more. Cheers to successful project management!
What is a clinical project manager template? A clinical project manager template is a pre-made document (or digital structure within Gridfox) that helps organise and guide clinical trials. It includes sections for planning, tracking, and managing various aspects of the project.
Why are templates important in clinical trials? Templates are important because they provide a structured approach, helping to ensure consistency and compliance with regulations throughout the trial.
How do templates make project management easier? Templates simplify project management by providing a clear framework for organising tasks, timelines, and responsibilities, making it easier to track progress and meet deadlines.
What are the key features of a good template? A good template should be easy to use, customisable, and comprehensive, covering all necessary aspects of the clinical trial process.
Can templates be customised for different trials? Yes, templates can be tailored to fit the specific needs and requirements of different clinical trials, allowing for flexibility in their application.
Are digital tracking tools better than paper-based ones? Digital tracking tools are generally more efficient than paper-based ones as they allow for real-time updates, easier data management, and better collaboration among team members.
See your entire business at a glance
Get a clear, all-in-one view of your entire business, so you can stay on top of everything that matters. Whether you're juggling multiple projects or just need a better way to stay organised, our platform gives you the visibility you need, fast.
Instant setup. No payment details needed.